
The Gibbs phase rule for nonreacting systems provides the most convenient method for determining how many intensive variables are important in phase equilibria. For example, the listing order L W > H > V > L HC means that hydrates (H) contain less water than the liquid water phase (L W ), but more water than vapor (V), which in turn contains more water than liquid hydrocarbon (L HC). The order of phase listing is by decreasing water concentration.
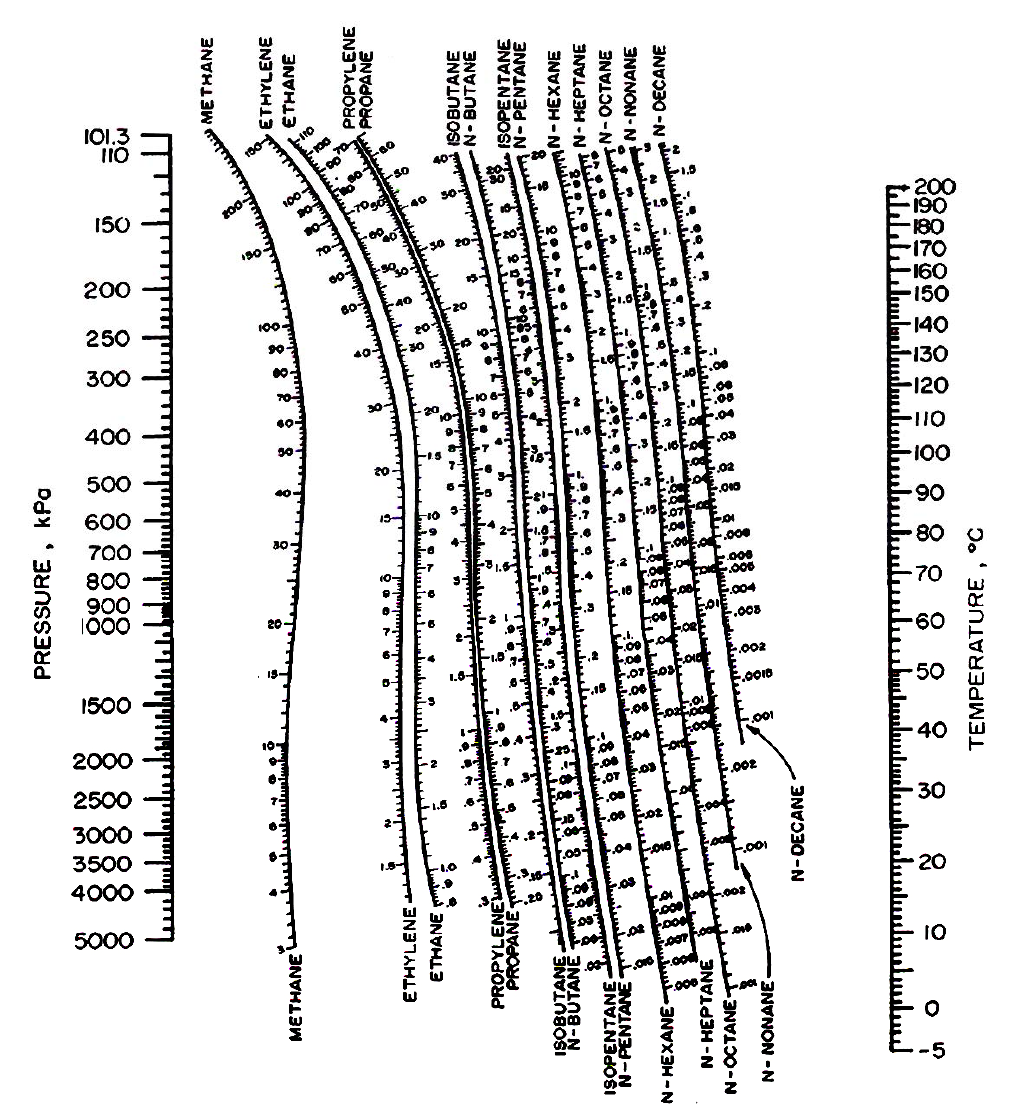
Phases are homogeneous regions of matter-gas, liquid, or solid-that can be analyzed using common tools such as pressure gauges, thermocouples, and chromatographs. A chapter on emerging technologies related to flow assurance and hydrates is in the Emerging and Peripheral Technologies section of this Handbook. therefore, hydrate-formation regions normally are avoided. They are solid crystalline compounds that typically plug flow channels, valves, drillstrings, blowout preventers, etc. This is an important section-hydrates are the most common solid-phase problem in flow assurance. This H 2O + hydrocarbon equilibrium without hydrates exists at high temperature or low pressure or when only large (greater in size than n-pentane) hydrocarbon components are present.īecause of the importance of hydrates in H 2O + hydrocarbon equilibria, however, the largest and third section of this chapter deals with systems containing small hydrocarbon molecules (<9Å) that form hydrates with water. The second section goes on to cover the simplest case-that of an H 2O + hydrocarbon mixture when all phases are fluid, as vapor and/or liquid, and without hydrate formation. Only the two most common concerns are treated in this section for a rigorous discussion of H 2O + hydrocarbon phase diagrams, see Harmens and Sloan. The first section covers phase definitions and the Gibbs phase rule, which are used to define the problem.

This chapter is divided into three main sections. Such a qualitative understanding and a few hand calculation methods serve as an initial check on the quantitative predictions of computer programs. This chapter also explains qualitative trends, to help the engineer to understand the implications of temperature, pressure, and composition changes. Quantitative predictions of macroscopic phase behavior are illustrated by example, along with a few results from hand calculations, though the many excellent commercial phase equilibria computer programs now available largely have eliminated the need for the hand calculations. This chapter discusses H 2O + hydrocarbon phase equilibria in macroscopic terms, such as temperature, pressure, concentration, and phase diagrams-more easily applied by the engineer-because a quantitative molecular prediction of H 2O + hydrocarbon phase behavior is beyond the current state of the art. Another example is the very high normal boiling point water has relative to its molecular weight. One example is water’s very high heat of vaporization, which absorbs large amounts of heat and buffers many hydrocarbon reservoir temperatures. Hydrogen bonds are responsible for most of the unusual properties water displays. Hydrocarbon molecules have a weak, noncharged attraction for each other, while water attracts other water molecules through a strong, charged hydrogen bond.īecause hydrogen bonds are significantly stronger than those between hydrocarbon molecules, hydrocarbon solubility in water (and that of water in hydrocarbons) is very small. This splitting of phases affects almost all treatments of H 2O + hydrocarbon systems and is caused by the different molecular attractions within water and hydrocarbons. When hydrocarbon contacts water, the two components separate into two phases in which the mutual component solubility is less than 1.0 mol% at ambient conditions.
Water generally is avoided because it is incombustible, and hydrate solids usually are avoided because their presence creates flow assurance difficulties. The phase behavior of H 2O + hydrocarbon mixtures differs significantly from the vapor/liquid equilibria of normal hydrocarbons in two ways: the aqueous and hydrocarbon components usually separate, with very low mutual solubility and hydrates often form with water and hydrocarbons smaller than n-pentane.


 0 kommentar(er)
0 kommentar(er)
